Passive Absorption
Passive Absorption (Passive Uptake):- It does not require expenditure of metabolic energy.
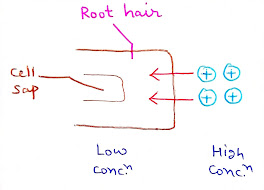
a. Simple Diffusion:- When the concentration of mineral salts is higher in the outer solution than in the cell sap of the root cells, the mineral salts are absorbed according to the concentration gradient by simple process of diffusion.
b. Ion Exchange Mechanism:- The ions adsorbed on the surface of the walls or membranes of root cells may be exchanged with the ions of same sign from external solution. For example, the cation K+ of the external soil solution may be exchanged with H+ ion adsorbed on the surface of the root cells. Similarly, an anion may be exchanged with OH– ion. There are two theories regarding the mechanism of ion exchange:-
i. Carbonic Acid Exchange Theory:- According to this theory, the CO2 released during respiration of root cells combines with water to form carbonic acid (H2CO3). Carbonic acid dissociates into H+ and an anion HCO3– in soil solution. These H+ ions may be exchanged for cations adsorbed on clay particles.
The cations thus released into the soil solution from the clay particles, may be adsorbed on root cells in exchange for H+ ions or as ion pairs with bicarbonate.
ii. Contact Exchange Theory:- According to this theory, the ions adsorbed on the surface of root cells and clay particles (or clay micelles) are not held tightly but oscillate within small volume of space. If the roots and clay particles are in close contact with each other, the ions adsorbed on clay particle may be exchanged with the ions adsorbed on root-surface directly without first being dissolved in soil solution.
c. Donnan Equilibrium Theory:- The membrane is selectively permeable in nature. Some are permeable to ions and some are impermeable.
The ions for which the membrane is permeable enter the cell by diffusion. This disturbs the equilibrium. To re-establish the equilibrium, there is an exchange of cations and anions again.
d. Mass Flow Theory:- Ions are also transferred along with the flow of water. Therefore, the absorption of mineral salts from the soil occurs in a water-soluble state. Higher the rate of transpiration, greater is the absorption of mineral salts.